CONNECTED 2025 || PARTNER: VIRIDIAN

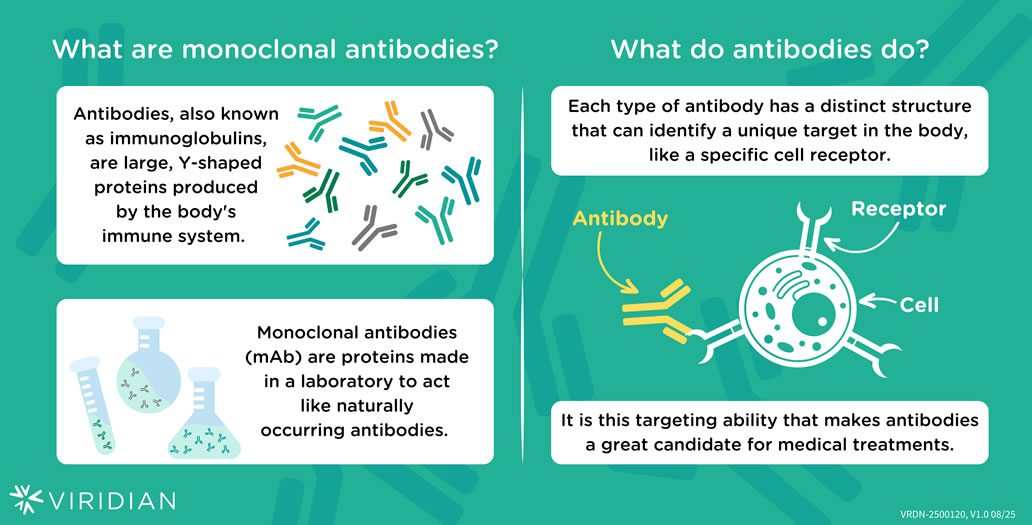
Viridian is a biotechnology company based in Waltham, Massachusetts focused on discovering and developing potential new medicines for people living with autoimmune and rare conditions.
Leveraging our team’s expertise in antibody discovery, protein engineering, and clinical development, Viridian is studying multiple potential new treatment options in clinical trials for people living with thyroid eye disease (TED).
Viridian’s Patient Advocacy & Engagement team serves as a bridge between Viridian and the patient community. If you are interested in learning more about Viridian or connecting with a member of our Advocacy team, please email
patientadvocacy@viridiantherapeutics.com or visit www.viridiantherapeutics.com.
Overview of Viridian’s Clinical Development Programs for People Living with TED
At Viridian, we are studying two potential treatments in late-stage clinical trials for people living with thyroid eye disease, veligrotug (formerly referred to as VRDN-001) and VRDN-003. Veligrotug and VRDN-003 are investigational study drugs. Neither has been approved for commercial use by any regulatory authority nor determined to be safe and effective for any use by a regulatory authority.
Both veligrotug and VRDN-003 are insulin-like growth factor-1 receptor (IGF-1R) inhibitors. The IGF-1 receptor and auto-antibodies against the IGF-1 receptor play a crucial role in the development and progression of TED. In the eye, IGF-1 affects growth and development, production of new cells, formation of new blood vessels, and inflammation. In the case of TED, there are an above normal number of IGF-1 receptors on the cells found in and around the eye, which leads to an increase in the cells’ activity (signaling) leading to inflammation and tissue growth. This expanded tissue pushes on the nerves, blood vessels, and the eye itself resulting in damage to the muscle and fat, redness of the eyes, pain, bulging of the eyes (proptosis), and double vision (diplopia).
With veligrotug and VRDN-003, we are looking to reduce the over-signaling of IGF-1R which causes the inflammation and tissue growth associated with TED and therefore improve symptoms and outcomes for people living with TED.
Veligrotug: A monoclonal antibody targeting the insulin-like growth factor-1 receptor (IGF-1R) that is administered through an intravenous (IV) infusion. A full treatment course is 5 IV infusions, given three weeks apart with an approximate infusion time of 30-45 minutes.
All of Viridian’s clinical trials for veligrotug have fully enrolled.
VRDN-003: A monoclonal antibody targeting IGF-1R. VRDN-003 has the same binding domain as veligrotug and is engineered to have a longer half-life. VRDN-003 also differs from veligrotug in that it is administered via a subcutaneous injection. A subcutaneous injection is an injection given in the fatty tissue, just under the skin.
Viridian is currently enrolling participants in clinical trials studying VRDN-003.
Enrolling Clinical Trials
- VRDN-003-303
- Safety and tolerability study of subcutaneously administered investigational drug VRDN-003 for people with active or chronic TED
- https://clinicaltrials.gov/study/NCT06812325
- Recruiting participants
- VRDN-003-304
- A randomized, open-label study to determine if the investigational drug VRDN-003 for people with active or chronic TED is safe and tolerable and to see how the body reacts when given through a subcutaneous injection using either an autoinjector or a vial and syringe
- https://clinicaltrials.gov/study/NCT07155668
- Recruiting participants
We are thankful for the many people involved in the various clinical trials, including those individuals living with TED who are participating, without whom this research would not be possible.
Viridian’s Responses to TED Community Questions
- What is your company’s focus when it comes to helping people living with TED and when did you start working in the TED space?
- Viridian has been working in the TED space since our founding. The company was founded in 2020 with a focus on developing potential new treatments for people living with TED and with conditions that are underserved by current therapies.
- What is the therapeutic approach for your investigational drug or currently approved treatment?
- The two investigational treatments we are studying as potential treatments for people living with TED, veligrotug and VRDN-003, are monoclonal antibodies that inhibit IGF-1R. With these two monoclonal antibodies we are looking to reduce the over-signaling of IGF-1R which causes the inflammation and tissue growth associated with TED and therefore improve symptoms and outcomes for people living with TED.
-
- What symptoms are you measuring the impact of the investigational treatment on in your clinical trials? What has been observed so far in these trials, both from an efficacy and safety side?
- In our clinical trials for veligrotug and VRDN-003, we are studying the effects of these investigational treatments on a number of signs and symptoms of TED. These include bulging of the eye (proptosis), double vision (diplopia), and inflammation. In addition, we are also studying the safety of these investigational treatments.
- Because veligrotug and VRDN-003 are investigational treatments that are being studied, they have not been determined to be safe and effective for any use by a regulatory authority. They are not available outside of clinical trials.
- We look forward to sharing the outcomes and our learnings from these trials with the community when we are able.
- Where can people go to learn more about your clinical trials and how can people find out if they are eligible to participate?
- For more information on our clinical trials, including participant eligibility criteria, please visit https://www.viridiantherapeutics.com/patients/clinical-trials/ or speak with a healthcare provider.
- How do patients’ voices and experiences influence your research and product development?
- The perspectives and experiences of people living with TED help us understand the barriers to and gaps in treatment, and your voice ensures that we are meaningfully addressing and prioritizing the needs of the patient community.
- One example of how we do this is our TED Patient Advisory Council, which we started in early 2024. During regular meetings, we learn from members’ experiences and seek their input on a range of topics. For instance, they provide feedback on our clinical trials and educational materials and advise us on ways to engage with the community.
- Our focus for working with the patient community also goes beyond developing and delivering potential therapies. Through community partnerships, we work together to educate and raise awareness about TED, advance research on patient experiences and preferences, and establish impactful initiatives that support patients and their families.
Resources
- What are Clinical Trials
- View graphic below
- PDF available on Viridian website
- Living with Thyroid Eye Disease
- View graphic below
- PDF also available on from Viridian website



The webpage is intended for educational purposes and does not constitute diagnosis, care, or treatment recommendations. Please speak with a healthcare professional for all medical questions.
Veligrotug and VRDN-003 are investigational treatments that are available for use only in the setting of clinical trials through regulatory agency authorization and have not been determined to be safe and effective for any use by a regulatory authority.
Page last updated October 1, 2025
VRDN-2500121
TED Community Organization
We Educate
We Support
We Connect
All those affected by Thyroid Eye Disease (TED)


